CMS has
released a set of toolkits for providers, states, and insurers to help the
health care system get ready to swiftly administer the COVID-19 vaccines and
certain monoclonal antibodies once they become available. The resources are
designed to increase the number of providers that can administer the products
and ensure adequate Medicare reimbursement for the administration. To know the
payment allowances and other related information for these products, keep
reading!
Medicare Part B Payment for COVID-19 Vaccines and Certain Monoclonal Antibodies during the Public Health Emergency
To help the health care system get ready to swiftly administer the COVID-19 vaccines and certain monoclonal antibodies once they become available, CMS has released a set of toolkits for providers, states, and insurers. These resources will help to increase the number of providers that can administer the products, as well as ensure adequate Medicare reimbursement for the administration, but at the same time, make it clear to private insurers and Medicaid programs that they have a responsibility to cover these products at no charge to beneficiaries. The payment allowances and other related information for these products are in the tables below.
Payment Allowances and Effective Dates for COVID-19 Vaccines and their Administration during the Public Health Emergency
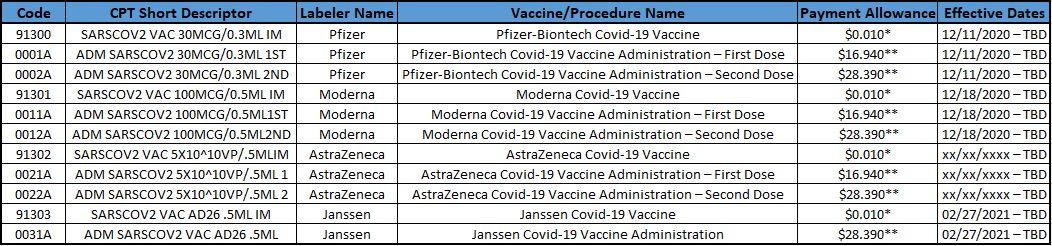
*It is anticipated that providers will not incur a cost for the product initially, so CMS will update the payment allowance at a later date. Providers must not bill for the product since they received it for free.
** For many providers, the rates will have to be geographically adjusted. Providers and suppliers with payments that are geographically adjusted by the methodology used by the Medicare Physician Fee Schedule (MPFS) should see files with the geographically adjusted payment rates for COVID-19 vaccine administration that are included in the “Additional Resources” section under the link. In some settings, other payment methodologies, such as payment based on reasonable costs, are used.
Payment Allowances and Effective Dates for COVID-19 Monoclonal Antibodies and their Administration during the Public Health Emergency
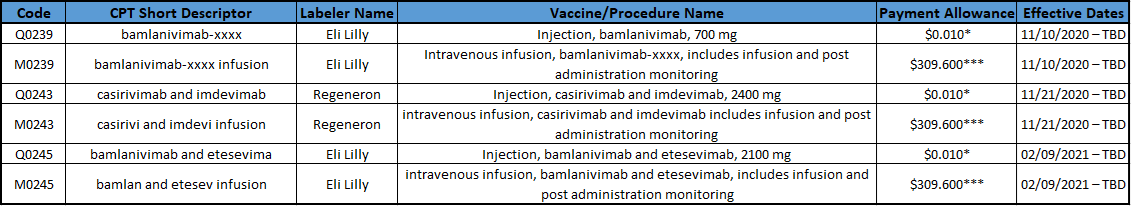
* It is anticipated that providers will not incur a cost for the product initially, so CMS will update the payment allowance at a later date. Providers should not bill for the product since they received it for free.
*** For many providers, Medicare will pay a rate of $309.60. However, the rates will also be geographically adjusted for many providers. Providers and suppliers with payments that are geographically adjusted by the methodology used by the Medicare Physician Fee Schedule (MPFS) should see files with the geographically adjusted payment rates for monoclonal antibody administration that are included in the “Additional Resources” section under the link. In some settings, other payment methodologies, such as payment based on reasonable costs, are used.



